Latest news in the TPD space
These are some recent developments in scientific publications, company news or other areas in the TPD space which caught my eye.
All views are my own and I do not represent or advocate for any biotech, investors or other entities.
I aim for my posts to accurate based on public domain information but if you see any errors or omissions, please alert me via the link below and I will endeavour to update accordingly
NB I'll sometimes post views on manuscripts posted on the preprint servers (BioRXiv & ChemRXiv) - please remember that these manuscripts have not yet been thoroughly peer reviewed so the data discussed should be interpreted accordingly
I feel the need...the need for speed
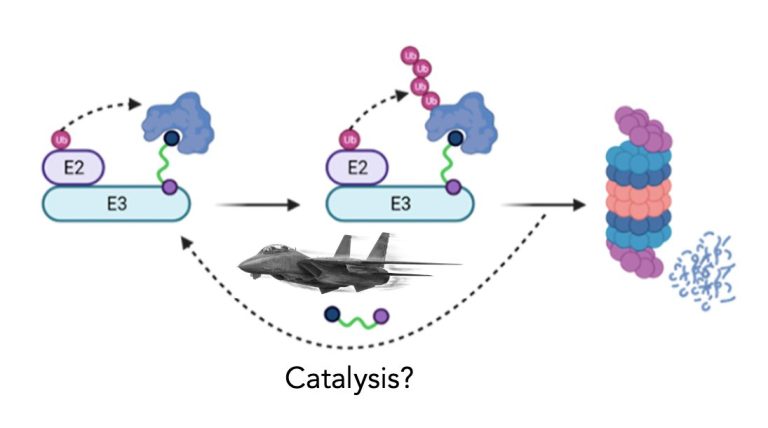
TPD drugs are catalytic. So what?
20th November 2024
One of the key advantages often cited for TPD drugs is their ability to utilize event-driven pharmacology. In other words, a single molecule of TPD drug, whether it’s a glue or bifunctional PROTAC, can mediate the degradation of multiple molecules of target in a departure from the “occupancy-driven” pharmacology which has underpinned drug discovery for well over 100 years ago.
So TPD drugs are catalytic. So what? Read on at my blog here.

TL;DR – Reflections on the state of play across the TPD field
13th November 2024
TL:DR – want to get a sense for the overall status of the TPD & Induced proximity field from discovery to clinic but have limited time? After spending a week at the 7th TPD & Induced-Proximity meeting in Boston recently, it allowed me to reflect on where we have gotten to. Read here for my distilled, 20,000 ft, helicopter, bullet point, executive summary view of where the TPD world stands today.
TPD is Big Business!
Here's all the companies you need to know
8th November 2024
I keep a personal database containing the 90 or so biotechs developing TPD and related induced-proximity-based therapies as a useful resource to help me navigate the increasingly complex field. It can be difficult to keep up to date given the rapid pace of progress in this field but the database shows some interesting trends in the field. To hear more about what the “TPDCo” landscape looks like and what it could all mean, read on at my blog here.


TPD meeting, Day 4: Looking to the future - new induced-proximity approaches to watch out for
29th October 2024
And that’s a wrap. The Boston TPD conference ended with a “Next Generation” day of a wide range of newer approaches to induced-proximity including some non-degrader plays. These strategies were all earlier in development but what they lacked in terms of clinical data, they more than made up for with excitement and potential. To read my take on some highlights, click here
TPD meeting day 3: the great science just keeps on coming
30th October 2024
So the mist came down this morning in Boston but the quality of the presentations at day 3 of the TPD meeting continued to shine out, and indeed the insight from many of the speakers lifted the clouds from a few thorny TPD topics. My post is a little late today as I had to watch the Dodgers clinch the World Series but for my 5 minute highlights, take a look here.


TPD meeting, Day 2: Over to the biotech folks to show us how to make TPD drugs
29th October 2024
Day 2 of the TPD meeting saw the biotech industry folks to the fore. Monday we saw the academics helping to unpick the basic science and now it’s on to the drug discovery teams who turn these ideas into clinical projects and patient impact – and they seem to be doing a pretty good job of it so far. To read my take on some highlights, click here
TPD meeting day 1: academic stars take centre stage
28th October 2024
Sunrise over Boston and the start of the biggest TPD meeting of the year. It’s not often that organisers of big international conferences give you carte blanche to organise a day of exceptional talks but I was given that opportunity to invite some of the best and most insightful scientists in the TPD field in today’s “New Frontiers in TPD & Induced-Proximity” opening day at the Hanson Wade 7th TPD & Induced-Proximity conference in Boston. And what a day it was – for a few of my personal highlights and thoughts on where the latest TPD innovations can take the field, read on here.

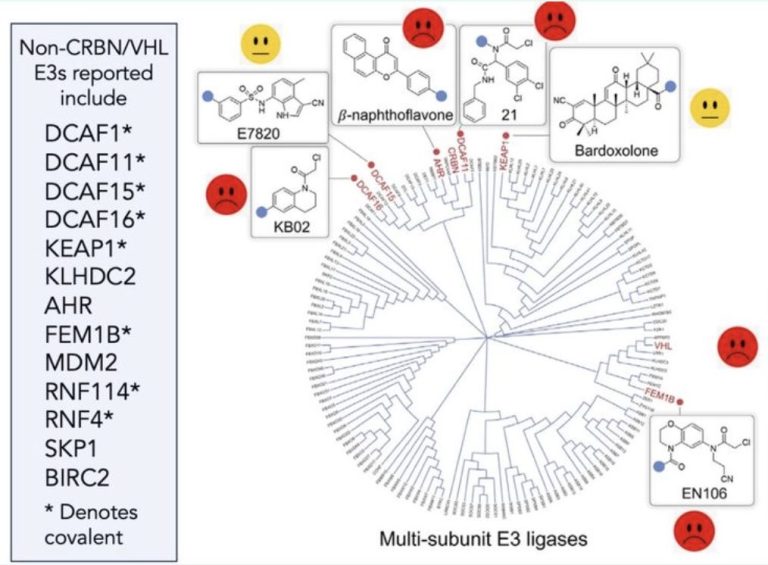
Not all E3 ligases are born equal. Are you feeling lucky?
23rd October 2024
“There are over 600 E3 ligases…” is a phrase you’ll see used all the time in TPD presentations and publications. So why is it we always default to using the same one or two in cereblon (CRBN) and von Hippel Lindau (VHL)?
There may be a few factors at play here which make CRBN & VHL particularly good at being repurposed to degrade your substrate of choice while other E3 ligases – well, they just don’t want to play ball and are just happy degrading their physiological substrate and probably not much else. - to read more, click here
Targets, targets, targets! How is TPD doing?
16th October 2024
I’ve been busy over the summer so my blog posting has suffered but now the weather is changing, I’ll do my best to get back to it!
When TPD exploded onto the drug discovery scene nearly 10 years ago it was accompanied with the usual hyperbole which often accompany shiny new things, namely that soon no drug target would remain undruggable as TPD would soon be degrading all those highly validated but hard to drug targets with the resultant tsunami of exciting clinical candidates. So, now we know more, how’s that been going?
Asher Mullard has just put out a great piece in Nature Reviews Drug Discovery which I share some thoughts on. To read more, click here.

TPD Conference Update and Summary of TPD Europe 20th-21st March
26th March 2024
Conference season is now in full swing and there’s plenty of opportunities to keep up to date with the latest in TPD & Induced-Proximity drug discovery work as well as catch up with colleagues at the wide range of meetings across Europe and the US. I’ve updated the conference page on my website so there’s now 13 TPD-focused conferences (not including the specialist sessions at ACS, AACR etc) in 2024. If there’s others I’ve missed please let me know and I’ll add.
I had the pleasure of chairing a number of sessions at last week’s TPD Europe meeting held in Kensington, London but for those of you not able to attend, I’ve summarised a few of my personal highlights - to read more, click here
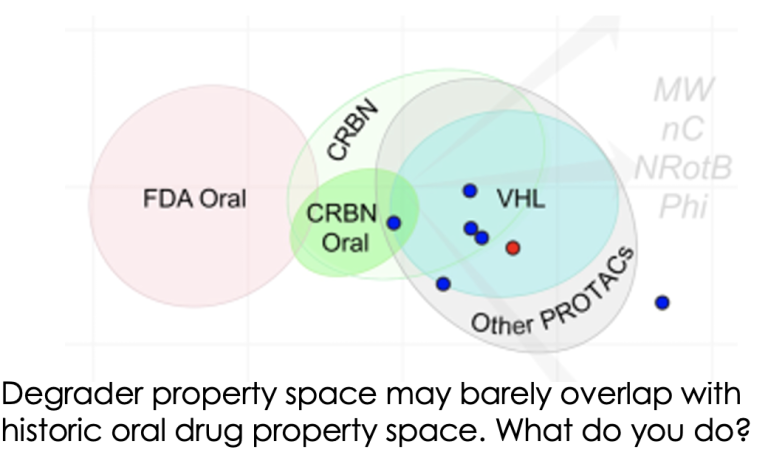
Dosing your degrader. To be or not to be.....oral? That is the question.
27th February 2024
Much has been written about how the properties of bifunctional agents including PROTACs are far outside the “usual small molecule space”. However, now we’re in the current renaissance of small molecule therapeutics including bifunctionals, peptides, macrocycles, conjugates etc, the infamous Lipinski Rule of 5 is feeling more like a mnemonic from a bygone age. Prompted by the recent Caron/Kihlberg Drug Discovery Today article on predicting how to design orally active PROTACs, just how important are the properties of degraders in determining what dosing routes are available to you? And indeed is oral dosing always preferable? To read more, click here.
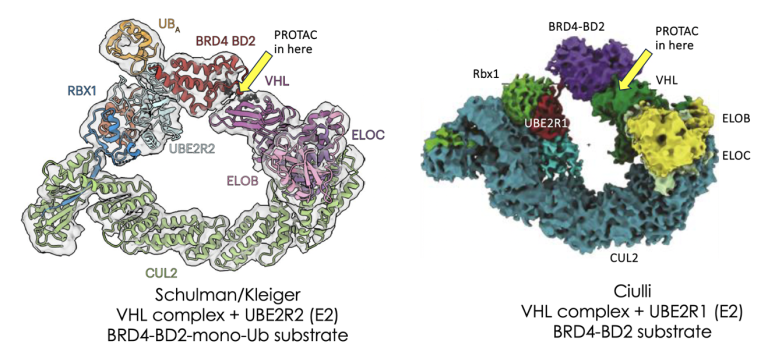
Welcome to the world of octonary complexes
19th February 2024
Just getting the hang of ternary complex modelling for TPD? Welcome to the world of octonary complexes
Much hard work is going into the prediction of ternary complex structures between E3 ligases, their substrates and small molecule PROTACs or glues which bridge the two. This is for good reason as understanding this interface is likely to lead to more stable ternary complexes which in turn can give improved degradation. However, formation of this initial ternary complex doesn’t always lead to degradation so we must look beyond the ternary complex to the wider ubiquitylation system which is exactly what a recent publication from the Schulman/Kreiger groups and a preprint manuscript from Alessio Ciulli and his team now start to do for VHL-dependent degradation. To read more, click here
Glues! Glues? Everywhere?
2nd February 2024
There is increasing excitement, and indeed success, around the search for molecular glues which induce target degradation, or potentially other, interesting downstream biology by persuading two proteins who don’t normally hang around together to do just that. This should perhaps not be surprising as nature uses molecular glues to regulate various biological systems (auxin, cyclosporine, FK506 etc) so it’s likely that HTS collections already contain many of these glues just waiting to be discovered.
Rationally designing small molecule glues which persuade two proteins to get cosy with each other is not trivial though many groups are now grappling with this challenge. The recent report from the Novartis team (BioRXiv preprint here) however shows that glues can also be found in more familiar places. To read more, click here.
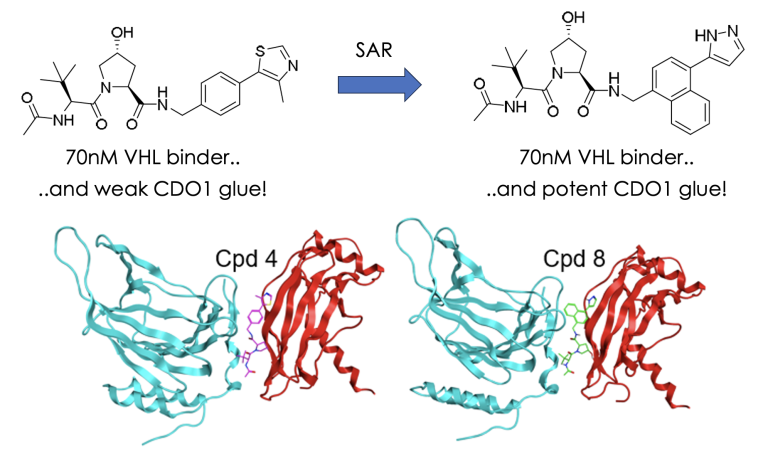

Presenting a quick guide to assessing new TPD agents
25th January 2024
There are increasing numbers of reports of new TPD agents using tried and tested mechanisms (eg cereblon or VHL E3 ligases) but also a plethora of new degrading approaches which recruit other E3 ligases or "effector" proteins. How can you tell which have the potential to be developed towards the clinic and which others may struggle? It’s not an easy question but I’ll try to give some pointers here by way of a simple beginners guide based on 3 tests inspired by a useful article published recently by Ingo Hartung from Merck and colleagues. To read more, click here
Agonizing over the best applications of induced proximity-based therapeutics
19thJanuary 2024
Although PROTACs & molecular glues are the currently best researched examples of proximity-based therapeutics, where perhaps counter-intuitively, facilitating (agonising) a substrate-E3 ligase interaction leads to functional antagonism and loss of protein function via proteasomal degradation, there are many other areas that induced-proximity is now showing potential. The Amgen team, led by Ryan Potts recently published a very helpful overview of the area which highlights some key factors and allows us to speculate where the field will find most success. To read more, click here.
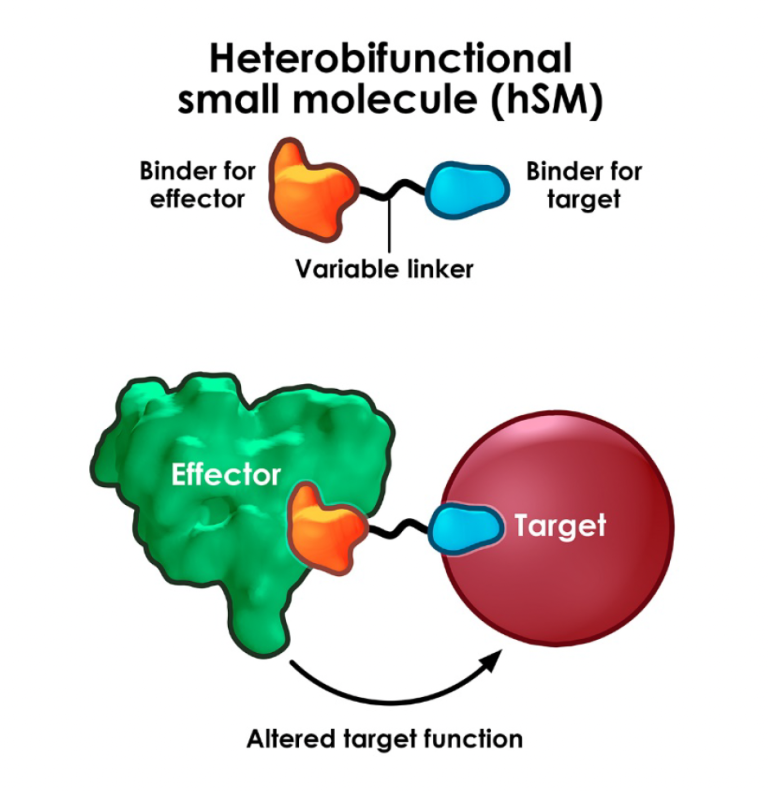
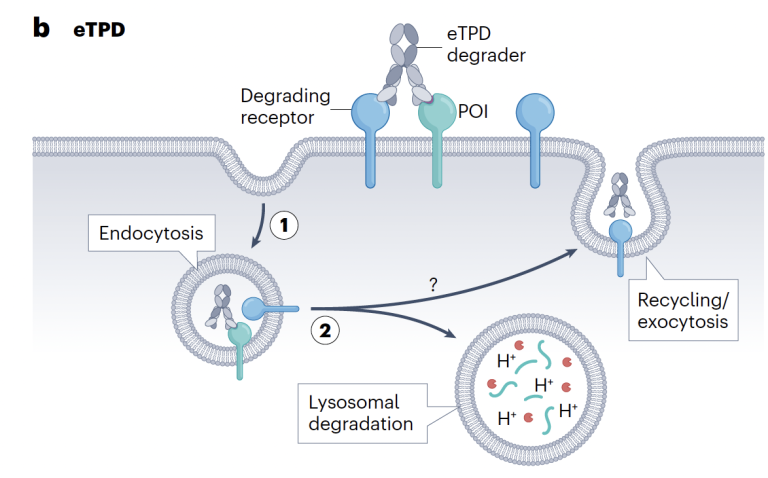
Extracellular degraders enter the fray
10th January 2024
Designing drugs to degrade intracellular proteins using PROTACs, glues etc has advanced at a dizzying speed with the first drug approvals now within sight but degrading extracellular or membrane-bound proteins has proved a somewhat tougher nut to crack.
A useful survey of the range of approaches to tackle these proteins recently appeared in Nature Reviews Drug Discovery, authored by Jim Wells (& his PhD sudent Kaan Kumru) who knows this space very well having published extensively and also co-founded TPD biotech EpiBiologics. Read more here.
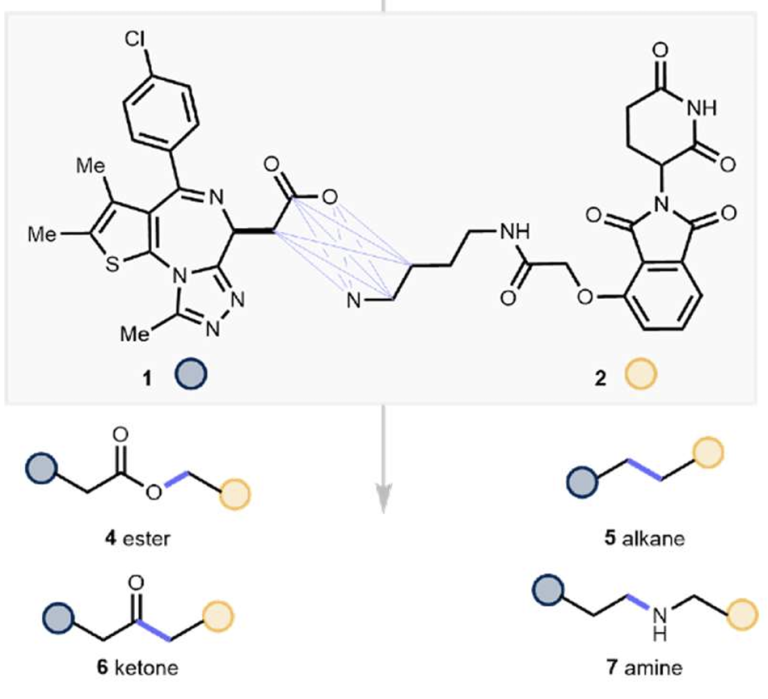
When acid + amine does not equal amide. Diverse TPD linkers now at your fingertips
1st January 2024
When making bifunctional molecules for TPD and other applications there can often be limited scope to vary the “end” ligands which bind the proteins to be brought into proximity. The linker however is where the magic happens and where the biological potency and drug-like properties can really be optimised so there’s a need for flexible chemistries which allow a wide range of linkers to be assessed quickly and easily. The recent U Michigan/Janssen preprint (here) does just that and is a great addition to the TPD synthetic toolbox. To read more, click here.

Covalent drugs may do more than meets the eye. Could they be molecular hand grenades or absolutely fine in moderation?
15th December 2023
There are many successful covalent drugs on the market and lots of TPD agents which seek to use this mechanism to their advantage though covalency is still a topic which seems to make some drug developers nervous due to the potential for unexpected, idiosyncratic events. The manuscript in BioRXiv from the Keriann Backus-led team at UCLA which first appeared in November 2023 suggests some wide-reaching consequences for use of covalent drugs which has caused some discussion in the field and so is worthy of a second look to highlight some potentially important caveats. Read more here.
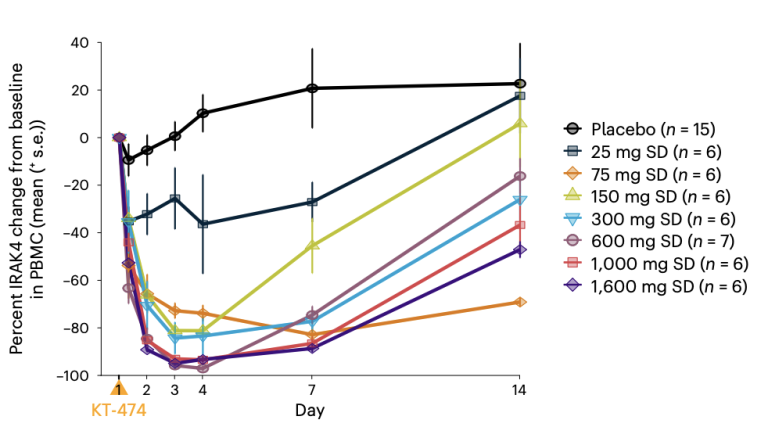
KT-474 - a case study in PROTAC clinical translation - so far, so good
7th December 2023
The summary of pre-clinical and clinical data of the Kymera IRAK4 PROTAC KT-474 published recently in Nature Medicine is a valuable example of a detailed analysis of activity of a new TPD agent – it underscores the potential of the TPD field, the potential of IRAK4 degraders to treat inflammatory disease and, perhaps most importantly in the longer term, the potential for TPD drugs to increase our understanding of whole body clinical pharmacology to a new level.
Kudos to the Kymera team for generating the data but then also for taking the time to summarise it in such an accessible format. Thanks guys. To read more, click here.
Finding new molecular glues that do "interesting" things
28th November 2023
Historically most molecular glue drugs were discovered based on their function. The details of the molecular glue mechanism were only added afterwards (~60 years afterwards in the most famous case of thalidomide). The words "molecular glue" and "serendipity" seem to have a strong affinity to each other. So new screening approaches to find glues could be very valuable.
The Winter group recently published an extension of their isogenic cell screening platform, married with cell painting to do just that. [to read more, click here]
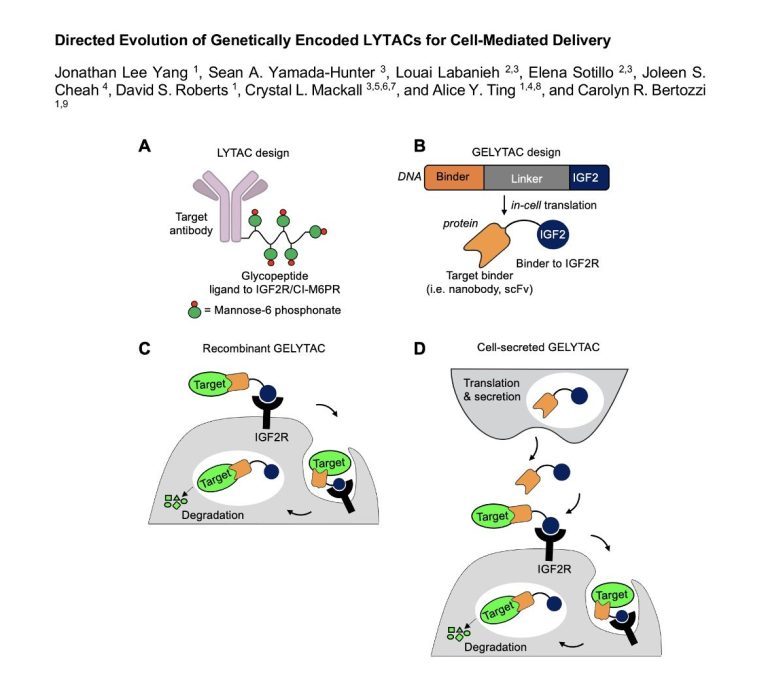
LYTACs to go? Deliver your degraders to order
20th November 2023
Drug targeting remains a hot topic to increase drug efficacy and reduce the potential for undesired systemic effects. In TPD space, much energy has been expended looking for tissue-specific ubiquitin E3 ligases which would give degradation of intracellular targets in specific cells with high expression of the E3 only. This has proved extremely challenging as there are very few E3s with suitable expression patterns and of those, persuading them to be recruited by bifunctional molecules as easily as CRBN or VHL to give potent cellular degradation has been very elusive indeed. Systemically administered LYTACs degrading extracellular targets via a lysosomal route have the same challenges of specificity but Carolyn Bertozzi's group may have a solution [to read more, click here]
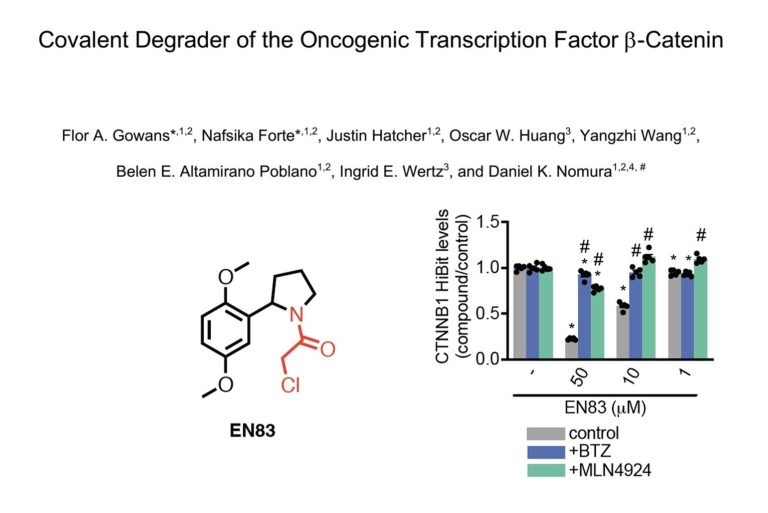
Another undruggable target falls? Let's wait and see
16th November 2023
For a long time the holy trinity of undruggable oncogenic targets were KRAS, c-myc & beta catenin. KRAS has famously shed it's undruggable tag and c-myc, with its lack of tertiary structure and fleeting nature is still proving tricky. Beta-catenin has also so far proved elusive leading drug discoverers to look elsewhere in the pathway but has Dan Nomura's group found a new way in? To read more, click here
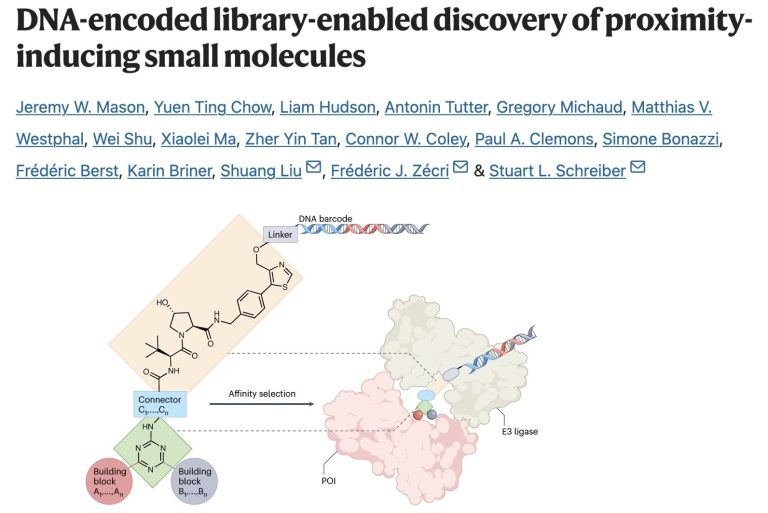
When DEL met TPD - a match made in heaven?
12th November 2023
DNA-encoded library (DEL)-screening has been around for along time now and allows the simultaneous screening of millions (or billions to trillions if you really want to though it has catches) of molecules to find binders to proteins. For protein degrading drugs finding a binder to your protein of interest (POI) is only part of the challenge [to read more, click here]

Degraders join the ADC party
6th November 2023
Everyone seems to want an antibody-drug conjugate (ADC) therapy right now. These drugs usually marry a tumor-selective antibody to a cytotoxic payload but several TPD companies think that using a protein degrading drug in place of the cytotoxic could give agents with improved activity. Like an ADC but actually termed a DAC, a degrader antibody conjugate [to read more, click here]
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.

